Inflammation Biomarkers in the Assessment and Management of Severe Asthma – Tools and Interpretation
Download the pdf document here: Biomarker Use in Severe Asthma Version 1 (20 June 2019; revised format)
Overview
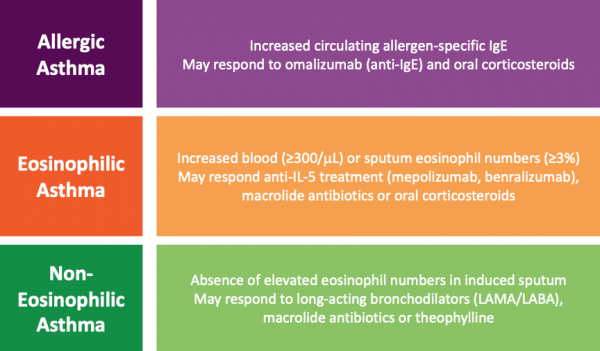
Asthma is a heterogeneous disease, meaning that there are many different subtypes. Groups of patients with similar disease characteristics can be clustered into asthma phenotypes. Well-recognised inflammatory phenotypes in severe asthma include allergic asthma, eosinophilic asthma and non-eosinophilic asthma (Figure 1).
In the severe asthma population, treatment shifts from a step-wise to a targeted approach. Disease pathology is caused by differing mechanisms in the different inflammatory phenotypes. Targeted therapy with monoclonal antibodies (mAbs) works by specifically blocking these disease pathways. Recognition and assessment of individual phenotypes is necessary to support a targeted therapy approach. Detailed assessment is required to inform the selection of targeted therapies which are likely to benefit individual patients.